EXPIRATION OR BEST BEFORE DATE?

As part of the EU Action Plan for the Circular Economy, an analysis was requested by the European Commission to EFSA to give greater clarity to the methods of indicating the expiration date or the Best Before date (BBE) by food chain operators and their understanding by consumers.
In this regard, it is important that Food Business Operators (FBO) follow an approach based on risk analysis when deciding:
- the type of date to be affixed (expiration or BBE)
- the shelf-life of the food and other information to be reported on the label to ensure food safety
This type of approach should be an integral part of the Food Safety Management System (FSMS) that food business operators are required to develop under current EU food safety legislation. In order to support these operators, and also to national authorities, an opinion has been published by EFSA to promote correct and consistent practices.
REGULATION (EU) N. 1169/2011
According to Regulation (EU) 1169/2011, in Art. 9 and Annex X, points 1 and 2, it is required that food labels (except in special cases) indicate an expiry date ("To be consumed by", "Use by") or a BBE ("Best before by / Best before the end"). The type of indication will be made on the basis of the perishable nature of the food and on the basis of the possibility that, after that date, there is an immediate danger to human health.
But, then, how to decide if, for a given food, a BBE or a expiration date is appropriate?
HOW TO DECIDE BETWEEN THE TWO
The decision to put an expiry date or a BBE in a food must be evaluated product by product after a thorough analysis of the characteristics of the food, the type of processing to which the food is subjected and the reasonably foreseeable storage conditions.
A Decision Tree (Figure 1) consisting of 10 sequential questions has been created to help Food Sector Operators (FBOs) in making this decision. The important assumptions underlying the CEO are:
- the decision is based on the presence of any pathogens at the end of the production process, and on the fact that they can grow or not or produce toxins during the shelf-life;
- in the absence of defined acceptable levels of pathogens, any significant growth during the shelf-life can increase the risk of disease in consumers and is therefore a fundamental parameter for the decision;
- heating / cooking, before consumption, may not be sufficient to eliminate all pathogens or their toxins: the treatment may in fact take place for insufficient times / temperatures. Furthermore, handling can carry a risk of post-cooking cross-contamination;
- in the case of the simultaneous presence of spores and vegetative cells of pathogens, the growth limits of the vegetative cells are applied, since these are wider than those for the growth and production of toxins of spore-forming species.
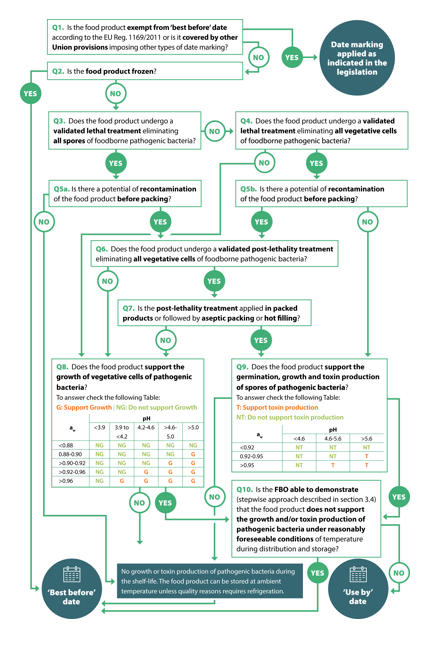
Fig. 1: Decision tree (published in the study cited at the end of this article) for choosing the date to be affixed to prepackaged foods to be stored at a controlled temperature
According to the decision tree, in the case of products that are processed in such a way as to ensure the absence of pathogens, or in the case of a processed product that does not allow their growth, the risk to the consumer does not increase during the shelf-life and the indication of a BBE is therefore appropriate.
Conversely, if there is no elimination phase of the pathogen or there is the possibility of recontamination after such treatment and, at the same time, the product allows its growth, the risk to the consumer is expected to increase during the shelf life and an expiration date is required.
SHELF-LIFE AND STORAGE CONDITIONS
In the event of an expiration date, the shelf life of a food should never exceed the shorter of "sensory shelf life" and "safe shelf life". The first relates to organoleptic changes in product quality, the second to food safety.
When it comes to shelf-life, it becomes mandatory to also talk about the reasonably foreseeable conditions of distribution, storage and use of food (Regulation (EC) No. 2073/2005): these are the conditions to which the food is exposed after it has left the immediate control of the FBO that produced it.
These conditions should include a certain degree of deviation from mere instructions, considering the habits of the average consumer, both during transport, storage and handling of the food. Currently there are no general guidelines in this area but only guidelines for laboratories regarding, for example, the shelf life of ready-to-eat foods in challenge tests.
In order to carry out an analysis that allows to validate the shelf-life of a food it is necessary not only to identify the pathogenic microorganisms and to know their initial concentration, nor is it sufficient to identify the characteristics of the food, the environmental ones and the possible effect due to the presence of other microorganisms in the food.
It is in fact also necessary to carry out an evaluation of the growth behavior of the pathogens under reasonably foreseeable conditions during storage, to determine the time at which they reach the maximum acceptable levels. For this purpose, the scientific literature, predictive microbiology models and / or laboratory tests (and in particular challenge tests) can and should be used on a case-by-case basis. Finally, trained and experienced staff with an in-depth understanding of food microbiology is required to perform appropriate shelf-life studies.
For more details, we recommend reading the study published in the EFSA Journal at the following link.

